The soil ecosystem
Miracle fertilizer
Fertilizer isn't literally a miracle, but it has been confused with one. In recent years Evangelical Christians have been converting Guatemalans away from Catholicism. The results, some Guatemalans say, are fantastic. Not only has drunkenness fallen, but people have been raised from dead. The fields even produce more food!
Did these religious conversions really reduce drunkenness? Perhaps. Did they raise people from the dead? Of course not. Did God, pleased with the conversions, bless the field so that it was more fertile? Probably not, considering that these conversions occurred at the same time chemical fertilizers were starting to became available to the region.
This story testifies to the efficacy of chemical fertilizers. They increase a field’s fertility so much that it is mistaken for a divine blessing! Divine or not, these fertilizers are indeed a blessing. Without modern nitrogen fertilizers we would be unable to feed 35% of today’s population. These 'modern' fertilizers we speak of are chemical fertilizers. Nitrogen fertilizer is made in large factories by using advanced chemistry and fossil fuels to extract nitrogen from the atmosphere and convert it to a form plants can consume. So successful are these factories that half of all the nitrogen in an American's body can be traced back to these fertilizer factories. Other fertilizers like phosphorus and potassium are acquired by mining deep into the earth.
N, P, and K
Plants need a variety of nutrients, but the main nutrient limiting the productivity of a soil is nitrogen (N), phosphorus (P), and potassium (K). This is why most fertilizers have only N, P, and K. Can a farmer apply only these three fertilizers to a field and maintain fertility forever? No, as plants have other nutrient needs. However, because these other nutrients are usually found in ample amounts in the soil, farmers can produce high yields using only N, P, and K fertilizers for decades—even centuries.
Lesser-nutrients
Ancient China once found itself a vassal of the Mongolians, paying tribute because it could not defeat Mongolian horsemen in battle. The Mongols had stronger, faster horses, while Chinese horses were much weaker. Two or more could be used to pull a chariot, but they could not be ridden by a mounted warrior. Why? According to historians, this is because the Chinese soils were deficient in the lesser-nutrient selenium, a nutrient needed for strong bones, while the Mongolian soils contained an abundance. The Mongols were thus superior in battle because their soils were superior in nutrients. Sometimes, it is the soil that makes the nation. For the Mongolians, their soil was their secret weapon.
Most of the time the word 'fertilizer' refers to plant available nitrogen (N), phosphorus (P), and potassium (K), yet there are many other nutrients essential for plants and animals. There is carbon, oxygen, and hydrogen that plants acquire from the air or water. Other elements, most of which are acquired from the soil, are ...
- calcium
- magnesium
- sulfur
- boron
- chlorine
- copper
- iron
- manganese
- molybdenum
- zinc(T2)
These nutrients other than N, P, and K are referred to throughout this lecture as lesser-nutrients. This is because they are needed in lower amounts relative to N, P, K, carbon, oxygen, and hydrogen.
There are some lesser-nutrients barely needed by plants, but are necessary for animal growth. Examples are selenium and iodine. Plants generally have little need for them, but animals do, and plants give us these nutrients by embedding it into the vegetation that animals eat. Plants can often be healthy and provide large yields, even if they grow in selenium- or iodine-deficient soils.
Consider iodine. All humans need it, but in low amounts. Mothers with excessively low iodine levels in their womb have children who score worse on literary tests, so just because you don't hear much about iodine doesn't mean it isn't a problem. Look at the label of the salt you buy and you will probably see that it also contains iodine (unless it is Kosher or pure Sea Salt). Since we all use small amounts of salt each day, the best way to ensure we consume small amounts of iodine is to add it to salt. Some individuals do not like the taste of iodine in salt and choose Kosher salt instead. The Nutrition Diva Monica Reinagel says this is okay, as, "Vegetables can be a source of iodine, depending on the iodine content of the soil in which they're grown."" Iodine may sound like a trivial concern, but recent research suggests that even with iodized salt pregnant women may not be receiving enough.
If a soil is being depleted of important lesser-nutrients, we could suffer the consequence. Many people in developing countries where iodine is not added to salt, and whose soils are lacking in iodine, suffer the consequences in the form of malnutrition from iodine deficiency. This is important, because the health of the plant doesn't tell us everything about its nutrient composition, which means a farmer can harvest a healthy crop without realizing it is deficient in iodine. Some minerals important to human health are not actually used by the plant, so the plants could be remain healthy even if its nutrient content for humans is in decline.
Although they don't get much attention in the U.S., lesser-nutrients are important. We don't hear much about them because they are usually so abundant in the soil that they pose no problem. One day, though, they might, so we should study them now instead of waiting until their absence causes a health problem.
Critics of chemical fertilizers rightly point out that N, P, and K aren't everything a plant needs, and farming without returning many of these lesser-nutrients to the field means you are basically mining the soil. How long can we continue to mine the soil? Some estimates for some specific locations suggests there are enough lesser-nutrients for hundreds, sometimes thousands, of years. Also keep in mind that nature naturally mines the soil. Virtually all plants acquire much of their lesser-nutrients by developing a symbiotic relationship with fungi growing around the plant roots. The plant captures carbon, hydrogen, and oxygen (the elements in sugar, so think of these as cookies) and brings them down to their roots to give to the fungi who need them. In turn, the fungi harvest various lesser-nutrients and transport them to the roots of the plant, who need them. So essentially plants are trading cookies for minerals of various sorts. The plants are using fungi to mine minerals, so it is really a big deal if humans mine the minerals themselves and give it directly to the plant?
Sometimes a lesser-nutrient problem exists even though it is prevalent in the soil. This is because not all nutrients are available to plants. North Dakota soils have large amounts of iron but much of it is unavailable because the soil's pH is too high. The solution is not to apply more iron but to lower the soil's pH, or to apply a chelate, which helps transport iron from the soil onto the surface of plant roots. Think of it like this. Hair is almost all protein, but humans can't digest it so there is no point in trying to eat it. Likewise, sometimes the pH of the soil prevents a plant from digesting a nutrient even though it exists in abundance.
We have an answer for what will happen when a soil becomes deprived of lesser-nutrients, because in a few areas it has already happened. In the last twenty years some wheat farmers in the state of Washington noticed that applying more nitrogen did not increase yields, which suggested the wheat's growth was limited by the absence of two other nutrients: chlorine and sulfur. How did Washington farmers respond? Fertilizer companies developed a market to profit off this need, and so farmers today easily acquire inexpensive chloride and sulfur, restoring high yields. When some other lesser-nutrient becomes scarce in the soil, fertilizer companies and farmers will respond in the same way. In fact, in areas like Oklahoma where lesser-nutrients are not a problem, there are fertilizer salespeople trying to sell fertilizer supplements. When agronomists test the performance of these supplements, they usually find they have no impact on yield. Now, just think how many salespeople would be marketing copper, iron, and boron, and zinc in Oklahoma if it was actually needed!
Fertilizer and the nutrient content of food
Some people have observed that the nutrient content of many foods has fallen over time, and they attribute this to a lack of lesser-nutrients in the soil. Is our reliance on chemical fertilizers causing our food to be less nutritious?
Research suggests the blame mostly belongs to new crop varieties. With the rise of chemical fertilizer, also came higher yielding crop varieties. These more efficient varieties of grains, fruits, and vegetables are subject to the genetic dilution effect, a concept describing the tradeoff between yield and nutrition. These improved varieties of plants achieve a higher yield in two ways (1) by taking more nutrients from the earth and/or (2) by packing less nutrients per pound of food. Most studies find that the falling nutrient content of food is due to the choice of new crop varieties, not a declining fertility of the soil.
This was illustrated nicely on the Broadbalk fields in England, where experimental plots have been maintained since 1843. Between 1843 and the 1960s, the concentration of lesser-nutrients in the wheat harvested remained steady, but then began falling, giving the impression that the soil was running out of zinc, iron, copper, and magnesium. However, the actual amount of [plant available] lesser-nutrients in the soil has remained steady or increased, leading researchers to conclude it was the choice of wheat varieties planted that reduced the nutrient content of the wheat, not soil deficiencies.
Of course, the nutrient content of food is less important than access to total nutrients, and over the last century the U.S. has increased its per capita production of almost every nutrient (and for the nutrients that are less available today, the decrease is small).
Figure 1—Percent change in nutrients contained in per capita US food supply compared to a century prior

Greater nutrient availability doesn't necessarily mean greater nutrient consumption, if patterns of food waste are changing at the same time more nutrients are produced. Studies of nutrient consumption in the UK find that the per-person intake of some lesser-nutrients like magnesium, iron, zinc, and copper has fallen over time, and in some cases is insufficient for a person's daily needs. While there are nutrient deficiencies in the U.S. they have not changed much since 1999. The purpose of the above figure is not to argue that there are no lesser-nutrient deficiencies in the U.S., but that the persistent use of chemical fertilizers does not seem to pose a nutrient problem.
Another way to inquire whether chemical fertilizers affect the nutrient content of food, is to compare non-organic food to organic food. Most of the time, non-organic food is raised using chemical fertilizers, whereas organic food must use other sources (e.g., manure, compost, crop rotations). Many scientific comparisons have been made, but the overall results suggest organic food is equivalent to conventional food in terms of nutrition. When grocery stores in the United Kingdom tried to market organic food as being more nutritious, it was ordered by the government to stop because it was considered false advertising. The stores could not refute the government's accusation, so they ceased advertising organic as nutritionally superior. Some more recent research finds that organic food may have more antioxidants, so perhaps future studies will deem organic food as more nutritious. Organic food does have less pesticide residues, but we defer this issue to the lecture on pesticides.
More than just dirt
If you want to frustrate a plant scientist, just refer to soil as 'dirt'. This drives them crazy, and for a good reason. Thus far I have been discussing crop production as if the soil was a mere container to hold nutrients like nitrogen—a mere dinner plate on which food is placed. A dinner plate is a very poor metaphor, though, because the soil is not a substance, but an ecosystem.
Just as health experts are learning all the ways our bodies are influenced by the micro-organisms in our intestine, plant scientists are discovering myriad ways that organisms within the soil affect plant growth. Plants often grow best when the soil is [almost] literally alive, containing a gallery of bacteria, fungi, worms, insects, and other critters. In just a teaspoon of soil there can be between 100 million and 1 billion bacteria! Tunnels dug by earthworms help aerate the soil. Some insects prey upon crop pests, thereby preventing crop damage. Some fungi settle on plant roots, helping plants communicate with one another by warning other plants of an approaching pest.
(If the idea of plants communicating surprises you, you have underestimated the complexity of plants, which can not only communicate, but have 15-20 different senses. Compare that to our measly five senses of smell, taste, sight, touch, and sound.)
There are some microbes that assist plants in acquiring nutrients from the soil. A bacterium named Paenibacillus can prevent tomatoes from being contaminated with salmonella. Other micro-organisms help assimilate carbon into the soil, thus reducing greenhouse gases concentrations in the atmosphere. There are bacteria living on the roots of legumes which snatch nitrogen from the air and convert it a plant-accessible for of nitrogen.
We should recognize that microbes are not always beneficial for crops. Sometimes they help the pests that damage crops, like the bacteria living inside the Colorado potato beetle. Normally, when the potato plant is being eaten, it recognizes the presence of a herbivore and erects its herbivore-relevant defences. However, the bacteria in the beetle tricks the plant into thinking that the beetle is a microbe, and so the plant erects the wrong type of defenses—and the beetle thrives. The job of agricultural scientists is to encourage the good microbs and discourage the bad.
Figure 2—The Soil Food Web

Many of these beneficial organism populations are reduced by chemical fertilizers, although the negative effects from such seem to be small. In cases where the effects are large, one can either reduce the use of chemical fertilizers or replace it with manure or compost. There is also the option of retaining the use of chemical fertilizers, but finding ways to compensate for the loss of organisms. For instance, the aeration of the soil normally performed by earthworms can be done with mechanical aerators instead. If fertilizers are killing large numbers of microbes, there are companies like Terra-One that sell fertilizer supplements that replace these populations (gardeners have been purchasing microbes for decades).
Healthy soils are high in organic matter, as it helps prevent erosion and retain moisture. Soils low in organic content can be improved by applications of manure or compost, or by the adoption of no-till methods, where the ground is never plowed and the roots formed by plants remain in the soil, undisturbed. Some farmers have actively sought to raise the organic matter in the soil, and have placed a monetary value on the organic matter at $3,775 per acre.
Just as some microbial colonies must be allowed to thrive naturally to preserve soil health, other microbes can be introduced into new areas to aid a degrading soil. Cropland under irrigation tends to suffer from salinity, as ground water contains levels of salt not present in rain ('salts' here does not mean NaCl, but go by a rather sophisticated chemistry definition). Excessive salinity changes the structure of the soil in a bady way and makes it more difficult for plants to absorb nutrients. This detracts from crop production, but some areas have no choice but to use irrigation. Another problem with high salt levels is that it prevents the formation of bacteria on legume plant roots, bacteria which can convert atmospheric nitrogen into a plant-accessible form. Researchers are optimistic that this problem can be mitigated by introducing bacteria from the Pseudomonas family into the soil, but salinity continues to be a problem.
Soil erosion
Soil may not be dirt, but a field that loses most of its topsoil essentially becomes dirt. Not only do the nutrients wash away with the topsoil, but the ecosystem goes with it. The dirt underneath topsoil has a different texture, and is usually less suitable for crop production. The point is that the topsoil is a resource, and we must conserve it as best we can. However, it is a lamentable fact that it is nearly impossible to raise crops without some soil erosion. Erosion is a natural part of life, even in the absence of humans, but crops are raised by plunging plows, cultivators, and/or planters into the ground, loosening the soil and leave it exposed, making it that much easier for rainstorms and winds to take it away.
Figure 3—Important terms

Nature has a defense against erosion. In a natural setting a soil will have a diversity of plants growing in it, and a diversity of insects, bacteria, and fungi as well. Most all of these soils will have an abundance of arbuscular mycorrhizal (AM) fungi, which produce a molecule called glomalin. This glomalin is a magnificent thing, for it acts as a glue that helps hold soil particles together. Soils that produce large amounts of glomalin also have more organic matter, and together these create pores in the soil that not only keeps the soil together but helps water percolate through, recharging aquifers, while the organic matter absorbs some of the water and stores it for future use.
This resilient system breaks down when the soil is disturbed through plowing or cultivating. The machines break the glomalin bonds, and exposing the sub-surface to the air causes the fungi and bacteria to act differently and releases carbon into the air. The soil glue is gone and the pores in the soil collapse, so that in heavily rainfall it carries the soil away through runoff instead of percolating below the ground. Without the organic matter the soil holds little water between rains. In short, every time the soil is disturbed it becomes less healthy.
Video 1—Ray Archuletta and the Slake Test (i.e., Soil Stability Test)
The key to reducing erosion is then to disturb the soil as little as possible and to try to always have plants growing, as the AM fungi and plant roots depend on each other. Erosion can be reduced to low levels by adoption of no-till planting methods where seeds are planted directly into an unplowed soil and weeds are managed using pesticides. The creation of transgenic soybeans, for instance, has made it much easier for farmers to control weeds with the herbicide Round Up, thus encouraging farmers to abandon the plow and adopt no-till methods which can reduce erosion by as much as 90%. Of course, this means greater pesticide use and reliance on transgenic crops, which some oppose. The point is that the soil is a valuable resource that must be protected, but such protection always comes at a cost.
There are ways of farming no-till without pesticides. One method developed at the Rodale Institute is to plant the field in a cover crop, like rye or vetch. A cover crop is a crop that is planted but not intended to be harvested. It will instead remain on the field to improve the soil. Some cover crops will be tilled into the soil to increase organic matter. Other farms just leave the cover crop in the field to die naturally, after which they will plant a new crop in the dead cover crop. The roots of these crops die but become organic matter matter for microorganisms, increasing the soil's carbon content.
Another alternative is to 'crimp' the cover crop near the end of its life, but before it produces viable seed. Crimping involves crushing the plant stem near its base to kill the crop in a way that keeps the plant attached to the soil. The stem is not severed, and is still attached to the root, but the plant is dead. The crimping mechanism should lay the plant down in the field at the same time. Below is a picture of me crimping a cover crop of rye at the Farm2Fork garden using a hand-held crimper (the same instrument used to create crop circles and fool farmers into thinking an alien landed in their field). This created a sheet of natural mulch covering the whole garden. I then planted vegetables in that field, and the use of the natural mulch allowed me to grow corn without tilling the soil or using herbicides to control weeds.
Of course, a working farm would use a mechanical crimper like the one below. Here, a crimper mounted on front of the tractor crimps and flattens a cover crop of vetch, and a no-till planter mounted on the back of the tractor plants corn seed. Under ideal conditions machinery will only need to enter the field three times during the year—to plant the rye, to crimp the rye and plant the corn, and then to harvest the corn—never once disturbing the soil.
Figure 4—Crimping cover crops for no-till agriculture

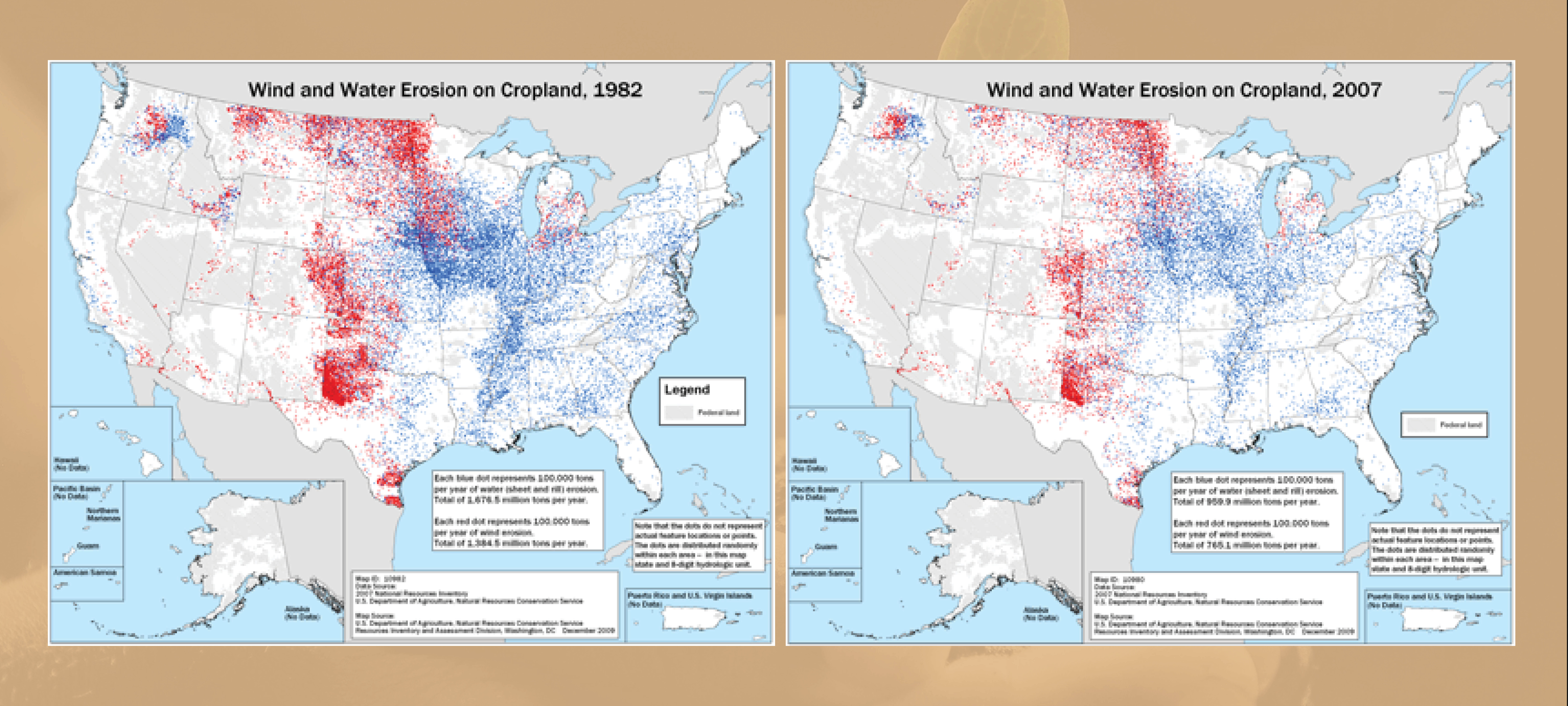
The figure below shows that erosion is indeed a national problem, but we are also doing better, as less soil was lost in 2007 compared to 1982. A major reason is indeed the adoption of transgenic crops, the pesticide Round Up, and various no-till production methods. When we debate whether we want to use synthetic pesticides and transgenic crops, we are also debating how much soil erosion we can tolerate. It is also the result of a change in culture. As technology makes no-till practices easier to implement farmers and agricultural scientists start thinking more about soil erosion, and the more we think about it, the higher we value that precious ecosystem that gives us life.
Figure 5—Soil erosion in 1982
(Blue and red dots represent 100,000 tons of annaul erosion due to water and wind erosion, respectively)
References
Albala, Ken. Food: A Cultural Culinary History [lectures]. The Great Courses. The Teaching Company.
Amaranthus, Mike and Bruce Allyn. June 11, 2013. “Healthy Soil Microbes, Healthy People.” The Atlantic. Accessed June 12, 2013 at http://www.theatlantic.com/health/archive/2013/06/healthy-soil-microbes-healthy-people/276710/.
Brandt, K., C. Leifert, R. Sanderson, and C.J. Seal. 2011. “Agroecosystem management and nutritional quality of plant foods: The case of organic fruits and vegetables.” Critical Reviews in Plant Sciences. 30:1-2:177-197, DOI: 10.1080/07352689.2011.554417.
Clifton, Vicki L, Nicolette A Hodyl, Paul A Fogarty, David J Torpy, Rachel Roberts, Ted Nettelbeck, Gary Ma, and Basil Hetzel. 2013. “The impact of iodine supplementation and bread fortification on urinary iodine concentrations in a mildly iodine deficient population of pregnant women in South Australia.” Nutrition Journal. 12(1):32 DOI: 10.1186/1475-2891-12-32.
Conniff, Richard. September 2013. “Enlisting bacteria and fungi from the soil to support crop plants is a promising alternative to the heavy use of fertilizer and pesticides.” Scientific American. Pages 76-79.
Charles, Dan. July 11, 2014. “Are Organic Vegetables More Nutritious After All?” The Salt. National Public Radio.
Davis, Donald R, Melvin D. Ep, and Hugh D. Riordan. 2004. “Changes in USDA Food Composition Data for 43 Garden Crops, 1950 to 1999.” Journal of American College of Nutrition. 23(6):669-682.
Davis, Donald R. February 2009. “Declining Fruit and Vegetable Nutrient Composition: What is the Evidence?” Horticultural Science. 44(1):15-19.
Dangour, Alan D, Sakhi K. Dodhia, Arabella Hayter, Elizabeth Allen, Karen Lock, and Ricardo Uauy. 2009. “Nutritional quality of organic foods: a systematic review.” American Journal of Clinical Nutrition. DOI: 10.3945/ajcn.2009.28041.
Dikötter, Frank. 2010. Mao's Great Famine. Walker & Company: NY, NY.
(D5) Deng, Shiping [soil biochemist at Oklahoma State University]. October 23, 2009. “Fertilizer Interaction with Soil Organisms.” SunUpTV. Accessed November 11, 2014 at https://www.youtube.com/watch?v=vas0mO4JnTE.
The Economist. October 8, 2009. “A no-brainer.”
The Economist. July 6, 2013. “Beans' talk.” Page 75.
Fan, Ming-Sheng, Fang-Jie Zhao, Susan J. Fairweather-Tait, Paul R. Poulton, Sarah J. Dunham, and Steve P. McGrath. 2008. “Evidence of decreasing mineral density in wheat grain over the last 160 years.” Journal of Trace Elements in Medicine and Biology. 22:315-324. DOI: 10.1016/j.jtemb.2008.07.002.
Germ, Mateja, Vekoslava Stibilj, and Ivan Kreft. 2007. “Metabolic Importance of Selenium for Plants.” The European Journal of Plant Science and Biotechnology.
Harl, Kenneth W. 2014. “The Han Emperors and Xiongnu at War.” The Barbarian Empires of the Steppes. The Great Courses. The Teaching Company.
Hager, Thomas. 2013. The Alchemy of Air. Broadway Books: NY, NY.
Homer. The Odyssey. Book XVII: Argument.
Huggins, David R. and John P. Reganold. July 2008. “No-Till: How Farmers Are Saving the Soil by Parking Their Plows.” Scientific American. Pages 68-77.
Intelligence2 Debates. April 13, 2010. “Organic Food Is Marketing Hype.”
Accessed October 1, 2014 at http://intelligencesquaredus.org/debates/past-debates/item/578-organic-food-is-marketing-hype.Kids World—Plant Nutrition. Plant Nutrients [web page]. North Carolina Department of Agriculture & Consumer Services. Accessed March 15, 2014 at http://www..ncagr.gov/cyber/kidswrld/plant/nutrient.htm
Leigh, G. J. 2004. The World’s Greatest Fix: A History of Nitrogen and Agriculture. Oxford University Press: NY, NY.
Logan, William Bryant. 1995. Dirt: The Ecstatic Skin of the Earth. Riverbead Books: NY, NY.
North Dakota State University. June 23, 2011. “Iron Deficiency Chlorosis in Soybean.” Crop & Pest Report. Accessed September 19, 2013 at http://www.ag.ndsu.edu/cpr/plant-science/iron-deficiency-chlorosis-in-soybean-6-23-11.
National Resource Conservation Service. December 4, 2008. Soil Erosion Enhancement Activity – SOE01 - Continuous No Till with High Residue. Enhancement Activity Sheet. Accessed March 10, 2014 at http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs143_007689.pdf.
Norwood, F. Bailey, Michelle Calvo, Sarah Lancaster, and Oltenacu Pascal. 2014. Agricultural Controversies: What Everyone Needs To Know. Oxford Publishing: NY, NY.
Pollan, Michael. December 23, 2013. “The Intelligent Plant.” The New Yorker. http://www.newyorker.com/reporting/2013/12/23/131223fa_fact_pollan?currentPage=all.
Rebagliato, M., M. Murcia, M. Alvarez-Pedrerol, M. Espada, A. Fernandez-Somoano, N. Lertxundi, E.-M. Navarrete-Munoz, J. Forns, A. Aranbarri, S. Llop, J. Julvez, A. Tardon, F. Ballester. 2013. “Iodine Supplementation During Pregnancy and Infant Neuropsychological Development: INMA Mother and Child Cohort Study.” American Journal of Epidemiology. 177(9):944. DOI: 10.1093/aje/kws333
Reinagel, Monica. 2011. Nutrition Diva’s Secrets for a Healthy Diet. St. Martin’s Press: NY, NY. Location 1263 in Kindle version.
Raun, William and Hailing Zhang. 2013. Data made available through personal communication. Both are plant scientists at Oklahoma State University.
Schnore, Jonathan. April 15, 2013. Personal Communication. Graduate Student. Department of Crop and Soil Sciences. Washington State University.
Schlegel, Alan. May 3, 2013. Personal Communication. Agronomist-in-Charge. Soil Management. Southwest Research-Extension Center of Kansas State University. Tribune, Kansas.
Seung Ho Chung, Cristina Rosa, Erin D. Scully, Michelle Peiffer, John F. Tooker, Kelli Hoover, Dawn S. Luthe, and Gary W. Felton. September 9, 2013 “Herbivore exploits orally secreted bacteria to suppress plant defenses.” PNAS. DOI: 10.1073/pnas.1308867110
Scholes, Mary C. and Robert J. Scholes. November 1, 2013. Science. 342:565-566.
Zhang, Hailin. 2013. Personal communication. Department of Plant and Soil Science. College of Agricultural Sciences and Natural Resources. Oklahoma State University.
TWAS, the academy of sciences for the developing world. October 6, 2013. “Salt-tolerant bacteria improve crop yields.” ScienceDaily. Accessed March 6, 2014 at www.sciencedaily.com/releases/2013/10/131006142707.htm.
(W1) Winter, Carl K. and Sarah F. Davis. 2006. “Organic Foods.” Journal of Food Science. 71(9). DOI: 10.1111/j.1750-3841.2006.00196.x
Williams, Alwyn, Gunnar Borjesson, Katarina Hedlund. 2013. “The effects of 55 years of different inorganic fertiliser regimes on soil properties and microbial community composition.” Soil Biology & Biochemistry. 67:41-46.
White, Jeffrey G. and Robert J. Zasoski. 1999. “Mapping soil micronutrients.” Field Crops Research. 60:11-26.
University of Wisconsin. January 11, 1999. Essential Elements for Plant Growth. Accessed May 10, 2014 at http://soils.wisc.edu/facstaff/barak/soilscience326/macronut.htm.